The main situations when IVF may be worth considering are:
- When the tubes are badly damaged and tubal surgery has less chance of success than IVF or in most cases where tubal surgery has already been unsuccessful. IVF should be considered because it bypasses the tubes.
- When the man’s sperm count is on the low side or abnormal, yet potentially capable of fertilizing an egg. Here IVF may be useful because fertilization can possible be manipulated and observed by the scientific team. This does not necessarily require sperm injection, or zona drilling, but simply very careful preparation of the sperm in suitable laboratory solutions.
- For certain women who have problems with the cervix perhaps ‘hostile’ mucus, IVF bypasses the cervix and its mucus.
- For women who are not ovulating spontaneously, but who produce eggs on fertility drugs without conceiving. In this situation, the ability to force the ovary to produce many eggs and then select the best ones for fertilization and transfer means that IVF may be suitable option.
- For some women with endometriosis or with very carefully investigated infertility which remains unexplained. We think that endometriosis is an excellent indication for IVF and have had particular success.
- For couples who have several factors together which are causing infertility; commonly a combination of poor male fertility and tubal disease are the most usual indications.
- Most recently, for certain couples who are at high risk of having genetically abnormal babies.
What to expect?
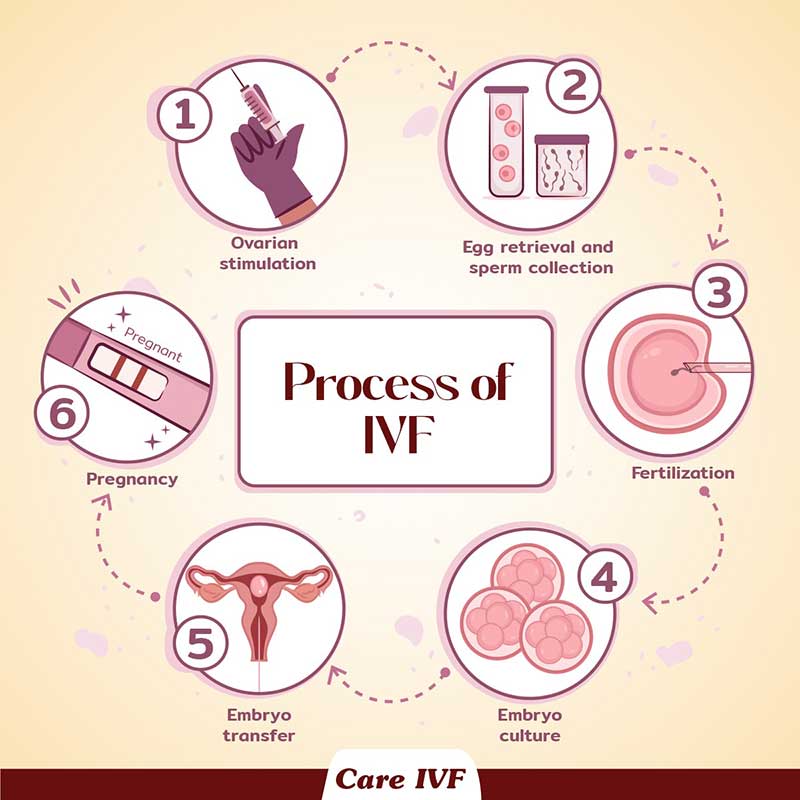
What happens during an IVF cycle?A typical IVF self stimulation cycle (involving wife’s eggs) at Care IVF starts at day 2 of menstrual cycle. It follows the 4 basic stages:
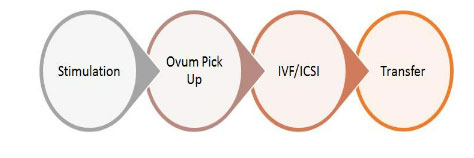
What happens during stimulations?
The day two of menstrual cycle is usually the day 1 of stimulation. This is when we start giving the stimulation injections.The typical IVF stimulation protocol follows the following steps:
Period day 2 = injections day 1:
| Period | Injection | |
| d2 - d5 | d1 – d4 | A baseline scan is done. The ovaries are assessed for cysts abd antral follicle count. Stimulation injections are started form d1 and continues till d4 |
| d6 | d5 | The patient’s e2 and progesterone levels are tested, and follicular scan is done. Based on the result the injection dosage may remain same or may be revised. This injection protocol continues till trigger. The husband’s semen sample is taken for freezing. This acts as a backup sample. |
| d7 - d8 | d6 - d9 | Stimulation inj + antagonist inj on day 6, if the follicle size is more than 13mm, antagonist protocol is started. |
| d10-d11 | d9/10 | Once the desired size of follicles are achieved (17-18 mm), trigger is induced by administering trigger injections. The opu should occur 36 hours from trigger. |
| d12-d13 | d11/12 | 36 hours post trigger the eggs are retrieved by oocyte retrieval. Husband is asked to deposit fresh sample on this day. IVF/ICSI is done on the same day within hours of pick up. |
Tthe pregnancy card test is done 9 days after (day 5) transfer and two weeks following this a usg tvs scan is done to confirm the positive cardiac activity.
If you are older, especially if you’re over 42, and have not succeeded with other therapies, or if you have premature ovarian failure (POF), also known as early menopause, your treatment options are limited. Egg donation, which involves the use of eggs donated by another woman who is typically in her 20s or early 30s, is highly successful. The woman receiving the donated eggs is referred to as the "recipient". The egg donor receives fertility medications to stimulate the production of multiple eggs in her ovaries. At the same time, the egg recipient is given hormone therapy to prepare her uterus to receive the fertilized eggs (embryos). After the eggs are obtained from the donor; they are fertilized in the laboratory with sperm from the recipient’s partner. Several days after fertilization, the embryos are transferred to the recipient’s uterus.
Donor-egg IVF offers a woman an opportunity to experience pregnancy, birth, and motherhood. The child, however, will not be genetically related to her but will be genetically related to the father and the egg donor. Many programs recommend counseling so that all parties in a donor-egg agreement understand the ethical, legal, psychological, and social issues involved.
We strictly adhere to the Egg Donor selection criteria laid out by Indian Council for Medical Research. The Egg donor goes through various Check points of screening before she is selected as an Egg Donor.
Donor-egg IVF offers a woman an opportunity to experience pregnancy, birth, and motherhood. The child, however, will not be genetically related to her but will be genetically related to the father and the egg donor. Many programs recommend counseling so that all parties in a donor-egg agreement understand the ethical, legal, psychological, and social issues involved.
We strictly adhere to the Egg Donor selection criteria laid out by Indian Council for Medical Research. The Egg donor goes through various Check points of screening before she is selected as an Egg Donor.
Check Points
- The Egg donors should be in the age group of 21 to 35 years. If her other basic criteria’s like qualifications, background, looks are matching the requirements she is okayed for the next level of screening, else rejected.
- The first screening test in the donor selection process is to see her Antral follicle count (AFC). This is done by a trans vaginal ultrasound scan and the follicles are counted. A good AFC count is indicative of good ovarian reserve and a good response to stimulation. If the Count is normal the next level of screening is done.
- The next step is to counsel the donor regarding the egg donation process. They are made to understand about the consents that they have to sign and that the risks or complications of this process. Psychological counseling is crucial and only when the donor understand and consents that we move to the next level of testing.
- AMH is a significant screening test. AMH levels help predict the ovarian reserve and tells us if we will have a good number of eggs on stimulation. Egg donors with AMH value of...or above are screened. The donor undergoes a series of blood tests that screen for any infectious diseases, HIV, Syphilis or Thalassemia. The donor’s medical history is taken and we also look for any family history of disorders.
Donor Matching, Special Donors
- The donor’s identity needs to be kept confidential by Indian law. No picture or identification details can be provided.
- The recipients will be given a donor profile that gives the information such as Age, weight, height, complexion, eye color, ethnicity, religion, qualifications. The results of the screening tests can also be shared.
- We often get request from couples to provide donors from their ethnic / religious background. We accept such requests and try to recruit donor accordingly. We always try to match the donor as closely as possible to the couple’s ethnicity and looks and religious preferences.
- Matching donors with certain features like extremely fair complexion, vegetarian, eye color cannot be always guaranteed and is expensive.
- Egg Donors with Non-Indian Ethnicity - For International Patients who would like to undergo IVF treatments at Care IVF, we will be able to source Egg donors of foreign origin and ethnicity if they are willing to meet the additional costs. We are tied up with a number of International Egg donation agencies that provide specialized Egg Donor matching services like matching ethnicity, Complexion, iris color.
- Once the egg donor is selected, the treatment can be planned - Cycle with fresh EggsThis kind of cycle needs a bit of advance planning. The recipient’s cycle will be synchronized with the Egg donor’s cycle. This is accomplished by administering a combination of two or three hormonal medications. Usually oral tablets alone are used to make the lining of the uterus without the use of injections. Because eggs are not formed, manipulating the cycle to shorten or lengthen it to match the donor cycle is easier.
- Cycle with Frozen Eggs - Alternately the couple can opt for a cycle with frozen eggs. The couple can select a donor profile and the frozen eggs will be retrieved form KSOB (Kolkata Surrogacy And Oocyte Bank).In case of frozen eggs being used, the semen sample is taken when the eggs are taken out from the liquid nitrogen cans. The semen and eggs are fertilized in a laboratory using in vitro fertilization.The embryo transfer normally occurs two-five days after the eggs were initially retrieved. A blood test will be performed 10 days later to determine if pregnancy has occurred.
What is the success rate of pregnancy using donor eggs?
The success rate will vary depending upon the quality of semen and the overall health of the recipient involved. The per attempt success rate is about 45%. However subsequent miscarriage is possible in about 10-15%.What are the legal concerns?
The Indian law clearly states that egg donation is legal. The woman receiving the eggs is the rightful mother of the child. Adoption is not needed. The egg donor at the time of donation itself relinquishes all rights to the future child.Frozen Embryo Transfer: Natural Cycle
Each cycle of FET, involves multiple steps, occurring at a specific time during a four-week period. An approximate timetable and overview are presented below.- Monitoring follicular development
- Monitoring for LH surge
- Documentation of ovulation
- Embryo transfer
- Hormonal supplements
- Pregnancy test
- Follow-up consultation
Monitoring of Follicle Development
Monitoring of follicle development is done on day 2 or day 3 of your period and then daily from day 9 onwards till ovulation is documented.Monitoring for LH Surge
As the growing follicle nears maturity, the level of the hormone LH in the blood and urine rises dramatically. This is known as the LH surge. For the purpose of frozen embryo transfer, we define the day of the LH surge as the day the blood LH >=17. It is important that the LH be monitored on a daily basis, as the frozen embryo transfer will be timed from the date of the LH surge. The timing of the embryo transfer will depend upon the stage at which your embryos were frozen. Embryos frozen at a more advanced stage of development (blastocysts) will be transferred later than embryos frozen at an earlier stage of development.Documentation of Ovulation
In addition to monitoring your LH, your physician may also confirm ovulation with ultrasonography through the time of ovulation. If ovulation does not occur, as evidenced by failure of the dominant follicle to collapse on ultrasound, then the frozen embryo transfer may be canceled. Alternatively, hormonal supplementation may be provided during the remainder of the transfer cycle. Depending upon the individual physician’s protocol you may also be treated with oral medications such as methylprednisolone and doxycycline prior to the transfer.Embryo Transfer
Embryos are thawed on the morning of the scheduled frozen embryo transfe. We usually transfer 1-2 embryos during each FET cycle. However, this number is flexible, and your inhouse doctor will discuss this issue with you. Excellent FET pregnancy rates occur in most cases with the transfer of one to two embryos, which also minimizes the risk of multiples. The transfer of more embryos may increase the likelihood of a multiple pregnancy, which increases the pregnancy risks for the woman and the babies.The embryo transfer is accomplished under ultrasound guidance which will require the bladder to be full. A small plastic catheter is passed gently through the cervix into the uterus. After waiting 1-2 minutes to allow any mild cramping to resolve, the embryos are deposited in the cavity along with a very small amount of fluid. No anesthesia is required for the embryo transfer. You will be discharged after resting for 20 minutes.
Hormonal Supplements
Patients undergoing FET may not require hormonal supplementation when we document normal follicular development and ovulation. Unlike the initial IVF-ET procedure during which the progesterone-producing granulosa cells are aspirated, those cells remain functional within the corpus luteum during your FET cycle. Progesterone supplementation may be administered to patients with ovulatory dysfunction or luteal phase inadequacy. In these cases, progesterone injections or suppositories begin before the embryo transfer and continue until the pregnancy test is performed.Pregnancy Test
We will usually perform a serum pregnancy test 9-12 days following the embryo transfer. If the test is positive, we may measure the serum progesterone level and recommend that you continue taking progesterone for several additional weeks. If the pregnancy test is negative, progesterone is discontinued and a period begins in a few days.Follow-up Consultation
If the pregnancy test is positive, we will perform a vaginal sonogram about two weeks later. At this point, we are able to identify the number of embryos and can often see a heartbeat in the developing embryo. The risk of pregnancy loss is low after this developmental milestone. If the FET procedure is unsuccessful, you should schedule a consultation with your inhouse dr to review the procedure and discuss further treatment options.Frozen Embryo Transfer: Using Hormone Replacement
The steps involved in FET with hormone replacement include:- Hormone therapy (Estradiol and progesterone)
- Embryo transfer
- Hormonal studies and pregnancy test
- Follow-up consultation
Estrogen Therapy
It is important that you start estrogen therapy on the first day of your period. A dose of estrogen is usually administered for 10-14 days, although shorter or longer cycles may be used, Estofert® is the most common form of estrogen we use. This is a pill containing 2 mg of estradiol, the same hormone produced by the ovaries. We will have you take one pill thrice a day for about a fortnight. After about 14 days of Estofert®, (your physician may vary the dose or duration of therapy) progesterone is added. This may be administered vaginally or as an intramuscular injection. Estofert® and progesterone are continued until the day of the pregnancy test (usually 9-12 days after embryo transfer). If the test is positive, these medications may be continued for several weeks. Depending upon the individual physician’s protocol you may also be treated with oral medications such as methylprednisolone and doxycycline prior to the transfer.Embryo Transfer
Embryo transfer is usually performed on the fourth to seventh day of progesterone therapy varying with maturity of your embryo. Embryos are thawed or defrosted on the morning of the scheduled frozen embryo transfer. We usually transfer 1 to 2 embryos during each FET cycle. However, this number is flexible, and your physician will discuss this issue with you. The transfer of more embryos may increase the likelihood of a multiple pregnancy, which increases the pregnancy risks for the woman and the babies.Embryo transfer is accomplished under ultrasound guidance which will require the bladder to be full. A small plastic catheter is passed gently through the cervix into the uterus. After waiting for 1-2 minutes to allow any mild cramping to resolve, the embryos are deposited into the cavity along with a small amount of fluid. You will be discharged after resting for 20 minutes. No anesthesia is required for the embryo transfer.
Hormonal Studies - Pregnancy Test
We will usually perform a serum pregnancy test 9-12 days after the embryo transfer. If the test is negative, hormone therapy is discontinued and a period usually begins in a few days.Follow-up Consultation
If the pregnancy test is positive, we will perform a vaginal sonogram about two weeks later. At this point, we are usually able to identify the number of embryos and can often see a heartbeat. The risk of pregnancy loss is low after this developmental milestone. If the procedure is unsuccessful, you should schedule a consultation with your physician. We will review the procedure and discuss further treatment options.What is Embryoglue?
It’s an embryo transfer media that contains among other things, high levels of a special component called “Hyaluronan”, also known as Hyaluronic acid. Embryo glue is designed specifically for embryo transfer to ease the embryo’s adhesion to the mucous membrane of the uterus.What is Hyaluronan?
Hyaluronan occurs naturally in your womb, fallopian tubes and ovaries. Studies have shown that it makes secretions from these organs stickier, aiding fertilisation and implantation.Studies show that Hylarunan’s secretions increases exponentially during the receptive stage and immediately declines the next day. This indicates the importance of hyaluronan in initial stages of implanatation.What does Embryoglue do?
Embryo glue mimics the composition of uterine secretions. Although the similarity in composition between the viscous embryoglue solution and the uterine secretions partly explains the improved implantation rates, it is the high levels of Hyaluronan in the embryoglue that makes the components of the embryoglue mixable with the uterine secretions. It also plays a major role in helping the embryo adhere to the endometrium, there by aiding implantation.The process of implantation has three stages:
Stage1 - Contact Stage:The high viscosity of the glue makes the content of the transfer medium mixable with the uterine fluid thereby enabling the embryo to stay within the uterine lumen. By enabling the embryo to stay in the uterine lumen it helps minimize the unwanted movement of embryos. Stage 2 - Adhesion Stage:
The embryo expresses cell surface glycoprotein called CD44. The CD44 is a receptor for Hyaluronan. CD44 receptor are also found in the cell wall of endometrium cells exclusively during the receptive phase. The Hyaluronan have pods on its surface that act as receptacles for these CD44 receptors to dock on.Therefore The Hyaluronan molecules effectively serve the purpose of linking the CD44 receptors on the embryo and CD 44 receptors of the endometrium, thereby tightening the contact between the two.Hyaluronan is naturally present in the Uterine Secretions as well as provided externally by the embryoglue.
Stage 3 - Implantation:
Embryoglue helps in implantation, it helps in cohesion between the cells of the embryo and endometrial lining of the endometrium. Once the embryo is able to settle into a suitable position the invasive part of implantation can begin.
When is it used?
On your embryo transfer day, your embryos are dipped into the ‘glue’. Then they’re placed in your uterus. The adhesive effect of the medium may help your embryos stick to your endometrium.THERE IS A YOUTUBE VIDEO.
What is ICSI?
ICSI or Intra Cytoplasmic Sperm Injection is a laboratory procedure developed to help infertile couples undergoing assisted reproduction due to male factor infertility. ICSI, a form of micromanipulation, involves the injection of a single sperm directly into the cytoplasm of a mature egg (oocyte) using a glass needle (pipette). This process increases the likelihood of fertilization when there are abnormalities in the number, quality, or function of the sperm. ICSI is generally unsuccessful when used to treat fertilization failures that are primarily due to poor egg quality.How is ICSI done?
Before ICSI can be done, mature eggs must be retrieved from the female partner during a standard IVF cycle. The male partner's semen sample is prepared in the lab to isolate as many healthy moving sperm as possible. After allowing the eggs to rest for 4-6 hours following their removal, the tight outer coating of cells (cumulus) is removed from each egg. Only then can we be sure the egg is mature enough to undergo ICSI. Immature eggs cannot be injected. However, they can be incubated for a further 4-18 hours and reassessed. If they mature during that time and sperm is still available, they can undergo delayed injection. Fertilization rates with delayed injection are not as good as with usual ICSI techniques.What are the indications for ICSI?
- Very low numbers of motile sperm.
- Severe teratospermia (abnormal sperms).
- Problems with sperm binding to and penetrating the egg.
- Antisperm antibodies thought to be the cause of infertility.
- Prior or repeated fertilization failure with standard IVF methods.
- Frozen sperm limited in number and quality.
- Obstruction of the male reproductive tract not amenable to repair. Sperm may then be obtained from the epididymis by a procedure called microsurgical epididymal sperm aspiration (MESA), or from the testes by testicular sperm aspiration (TESA).
- Embryo biopsy for genetic testing of embryos can only be done if a sigle sperm is used to fertilize the oocyte thus requiring ICSI.
What are the fertilization rates with ICSI?
Fertilization occurs in 50% to 100% of injected eggs. The ICSI process may damage a small percentage of eggs but that usually happens in eggs which are bad anyways. The fertilized egg may fail to divide, or the embryo may arrest at an early stage of development. Approximately 40% of all ICSI cycles performed result in a live birth, which is comparable to rates seen with traditional IVF. Younger patients may achieve even more favourable results. Factors such as poor sperm / egg quality and advanced maternal age may result in lower rates of success.Are there any complications with ICSI?
ICSI does not increase the incidence of multiple gestations as compared to standard IVF. To date, there is no convincing evidence that the incidence of birth defects is any different with ICSI or IVF as compared to those children born to other parents of similar age and health. This is an area of ongoing investigation. Because some causes of male infertility are familial and are related to genetic problems, male offspring might have reproductive problems as adults. Despite these concerns, ICSI is a major advance in the treatment of severe infertility.What is LAH?
Laser Assisted Hatching is a laboratory procedure that is sometimes done along with in vitro fertilization (IVF) treatment. While the embryo develops, it is surrounded by cells that make up a protective shell (zona pellucida). The embryo naturally breaks out of this shell as it grows. Occasionally, the doctor may ask the laboratory to make a small "crack" in the outer shell of the embryo right before it is placed into the woman’s body (assisted hatching). The hope is that assisted hatching might help the embryo expand, implant into the uterine wall, and finally lead to a pregnancy.How is assisted hatching done?
During assisted hatching, the outer shell of the embryo is artificially weakened by making a small hole in the zona pellucida. This can be done in several different ways. The method we use and which is the most advanced method involves the use of a laser to "crack" the shell.Can assisted hatching cause problems with my embryo or pregnancy?
Rarely, assisted hatching can damage the embryo, making it unusable.The risk for identical twins might be slightly increased when assisted hatching is applied. Medical complications are higher in identical twin pregnancies than in normal, singleton pregnancies.
Will I benefit from Assisted Hatching?
Experts do not recommend the use of assisted hatching in all patients undergoing IVF treatments to conceive. Studies suggest that assisted hatching might help improve pregnancy chances for certain groups of patients. Assisted hatching may help improve pregnancy chances in women who have failed to get pregnant in previous IVF cycles and those with a poor prognosis (women who are older than 38 years or who have average or bad quality embryos).What are Chromosomes? What kind of abnormality can they have?
DNA is the blueprint of life. DNA carries in it small units called the genes which are the heritable blocks of life. The DNA in each individual is packaged into thread like structures inside each of our cells. These structures in which the DNA is packaged into are called “Chromosomes”. For each species there is a unique number of chromosomes. In humans this number is 46. Out of the 46 chromosomes there are two which determine the sex of the organism and are named as X and Y. These are called the sex chromosomes. A person who inherits XX would be a female while a XY individual is would be male. Rest 44 chromosomes are called the autosomes. Each of the chromosomes has a sister copy which is its exact duplicate. Thus, there are 22 pairs of autosomes in addition to a pair of sex chromosome. Each of the chromosome and its duplicate copy are identified by a unique number from 1 to 22. Each of the 22 pairs of autosomes and 1 pair of sex chromosomes have their own features and characteristics. Their unique structure and character makes them be identified separately. Thus, we have 22 pairs of autosome along with X and Y chromosome. This makes 24 unique chromosomes in the human cell. Hence the screening technique used in Pre-implantation Genetic Screening (PGS) gets its name as "24 Chromosome screening array".What is pre-implantation genetic screening (PGS)?
IVF /ICSI in patients results in a number of fertilized embryos. Although, the good embryos are chosen on basis of morphology, the implantation rates and subsequent pregnancy are not as high as you would expect. This is because embryos can have chromosomal anomalies or could be mosaics. Mosacism means that the embryo might harbour two type of cell population where one is normal and the other has some chromosomal abnormality. Low level mosaics are considered viable, however high level mosaics would look normal by physiology but fail when implanted.Knowledge about the aberration (structural or numerical) in the embryo would thus significantly increase the probability to implant a good embryo. In Preimplantation Genetic Screening (PGS), an aneuploidy screening of the embryo is performed. The embryo is probed for each of the 24 chromosome with the help of the unique probes or control chromosomes. This helps in determining any gross numerical aberrations that a morphologically healthy looking embryo might acquire. Thus, by PGS the care provider can choose a better embryo over the bad ones with respect to aneuploidy thereby decreasing the chance of an implantation failure.
PGS ensures that certain genetic diseases due to numerical aberration of the chromosome like Down’s Syndrome can be avoided.
Is PGS for me?
A genetic screening is always a safer way to select an embryo for implantation.We recommend genetic screening of embryos, particularly if:
- You are over 35 and have a higher risk of having a baby with a chromosome problem (such as Down’s syndrome)
- You have a family history of chromosome problems
- You have a history of recurrent miscarriages
- You have had several unsuccessful cycles of IVF where embryos have been transferred
- Your partner’s sperm is known to be at high risk of having chromosome problems.
How does PGS work?
The procedure for PGS is likely to be as follows:- You undergo normal IVF treatment to collect and fertilise your eggs.
- He embryo is grown in the laboratory for two - three days until the cells have divided and the embryo consists of about eight cells.
- A trained embryologist removes one or two of the cells (blastomeres) from the embryo.
- The chromosomes are examined to see how many there are and whether they are normal.
- One, two or three of the embryos without abnormal numbers of chromosomes are transferred to the womb so that they can develop. Any remaining unaffected embryos can be frozen for later use. However, embryos that have been biopsied may not be suitable for cryopreservation and use in subsequent treatment cycles.
- Those embryos that had abnormal chromosomes are allowed to perish.
Possible variations to this procedure
Testing at five – six daysIt is possible that instead of removing and testing one or two cells from a two – three day old embryo, some centres may allow the embryo to develop to five - six days, when there are 100-150 cells. More cells can be removed at this stage without compromising the viability of the embryo, possibly leading to a more accurate test.
Comparative Genomic Hybridisation (CGH)
A small number of clinics are now using a procedure called comparative genomic hybridisation (CGH) which allows centres to test for abnormalities in all 24 chromosomes. Comparative genome hybridization involves screening the unique regions of each chromosome with numerous unique chromosome specific markers and compared with a standard set of chromosomes probes. In this process fluorescent probes are used to screen the 24 chromosomes. Any difference or deviation in chromosomal content from the standard or control would be detected by change in the fluorescence intensity. The use of so many probes increases the resolution or chance to detect an anomaly if present. This array used is known as 24 sure. PGS testing with array CGH is 98-99% accurate.Is it better to test at day 3 stage or day 5 stage?
It has been conclusively proved that testing at Day 5 is better since the embryos are thought to be better if they survive till day 5. Also the problems with embryo mosaicism (see below) are lesser on day 5. Lastly chances of damage to the embryonic cells are minimal on day 5.However most clinics are not able to employ day 5 testing since it requires:
- A highly trained embryologist capable of doing biopsy on a day 5 embryo.
- Perfect laboratory (IVF culture) conditions so that the embryo can grow to day 5, otherwise the embryos will not grow because of poor environment.
- Good freezing facilities not being available since after day 5 biopsy, the embryos would need to be frozen and then transferred in another cycle.
What method of testing is better for embryos, FISH or aCGH?
aCGH is now widely used for preimplantation genetic screening (PGS) to detect gross abnormalities in the number or structure of chromosomes in an egg or an embryo.With CGH, a sample taken from an egg (the oocyte polar body) or a single cell of a blastocyst stage embryo is compared to a normal control or standard set of probes. Deviation in the DNA content due to chromosomal abnormality would be reflected in the fluorescent intensity. The currently used 24 sure aCGH is based on “microarray” techniques, thus increasing the probability of anomaly detection than the “standard” PGS. PGS testing with array CGH is 98-99% accurate.
Standard PGS uses techniques known as fluorescence in situ hybridisation (FISH). In this techniques fluorescent probes for chromosomes X, Y , 7, 13, 15, 16 ,18, 21and 22 are used to hybridize with the chromosomes in embryo. The probes are designed from a certain specific region of the chromosomes and the entire length of the choromosomes is not covered. Moreover, this method limits the use of 5 chromosomal probes for one slide due to limitation in the capacity of the scanning microscope to detect signals. Thus, FISH has lower resolution or power to detect a chromosome as the probes are designed from a specific region. Moreover it is limited to a number of 12 chromosome probes and hence do not screen for all the 24 as in case of aCGH.
What is my chance of having a baby with PGS?
Chromosomal abnormalities in the embryo are the most frequent cause of recurrent early miscarriages and recurrent implantation failure in IVF despite transfer of best quality embryos morphologically.It is said that even if the eggs are sperms are taken from normal healthy young couples, almost 40% of embryos will be genetically abnormal. If the woman is older, has bad quality eggs or if the man has poor sperm quality, 60-90% of the embryos may be abnormal.
The miscarriage rates has been shown to drop from 33.5% to about 6.9% in studies while the IVF success rates been shown to increase from 40% to about 60% when the above technique is employed.
What are the risks of PGS?
Some of the risks involved in PGS treatment are similar to those for conventional IVF. Some other problems unique to PGS treatment include:- Some embryos may be damaged by the process of cell removal
- Possibility that no embryos are suitable for transfer to the womb after PGS.
Mosaic Embryos
PGS relies on the theory that all the cells in a human embryo are chromosomally identical, so that if you examine one cell from an embryo, it will show whether or not all the other cells have a chromosomal abnormality.However, research has shown that in some embryos (known as mosaic embryos), the cells are not chromosomally identical. As a result, many such embryos will be discarded that are in fact capable of producing a normal pregnancy.
What is PGD?
Genes are passed on from one generation to another. The phenotype or trait of a gene depends on the genotype. Genotype can be described as a particular combination of the gene on the chromosomes. Depending upon the inheritance pattern of the genotype, a certain combination or variation of the gene can be harmful resulting in a disease phenotype. It can be traumatic to undergo an abortion if the baby is detected prenatally for some life threatening genetic disease. In families where there is a clinical history of such genetic diseases it very important that the disease causing combination is excluded in order to have a healthy baby. Preimplantation genetic diagnosis or PGD is such a platform where the disease causing gene combination is probed and embryos harbouring such combinations are excluded. Thus, even in families which manifest fatal genetic disorders, PGD gives a chance to have a healthy baby.Is PGD for me?
Genetic testing of your embryos may be recommended if:- You have had a number of abortions because your baby had a genetic condition
- You already have a child with a serious genetic condition
- You have a family history of a serious genetic condition
- In case of certain cancer like breast or ovarian cancer
Which clinics can carry out PGD?
A clinic must:- Have a genetic license
- Have a full time embryologist trained to carry out embryo biopsy
- Have an IVF laboratory suitable for advanced culture conditions
What are the risks of PGD?
Most of the risks involved in PGD treatment are similar to those for conventional IVF.With PGD, there is also the possibility that:
- Some embryos may be damaged by the process of cell removal
- Testing may not be 100% reliable or conclusive
How does PGD work?
The procedure for PGD is likely to be as follows:- You undergo normal in vitro fertilisation (IVF) treatment to collect and fertilise your eggs.
- The embryo is grown in the laboratory for two - three days until the cells have divided and the embryo consists of around eight cells.
- A trained embryologist removes one or two of the cells (blastomeres) from the embryo.
- The cells are tested to see if the embryo from which they were removed contains the gene that causes the genetic condition in the family.
- Embryos unaffected by the condition are transferred to the womb to allow them to develop.
- Any suitable remaining unaffected embryos can be frozen for later use. Those embryos that are affected by the condition are allowed to perish.
- About two weeks after the embryo transfer, the woman is given a pregnancy blood test.
Trophectoderm Biopsy
It is possible that, instead of removing and testing one or two cells from a 2-3 day old embryo, some centres may allow the embryo to develop to 5-6 days, when there are 100-150 cells.At this stage, cells within an embryo have separated into two types: cells which will form the fetus (inner cell mass) and cells which will form the placenta (trophectoderm). More cells can be removed at this stage (from the trophectoderm) without compromising the viability of the embryo, possibly leading to a more accurate test.What is the chance of having a baby with PGD?
It is difficult to assess success rates for PGD because there is currently little data available. Most women use this treatment not because they have fertility problems but because they want to avoid having a child with a genetic disease.As with most fertility treatments, success depends on many factors, including the woman’s age and whether a cause of infertility has been identified.Contents © 2025, Care Fertility Solutions Pvt Ltd. All Rights Reserved.
Design By: AV Solutions


